

On October 8-10, 2024, the 35th CPhi Milan 2024 (World Pharmaceutical Raw Materials Exhibition) was grandly opened at the Milan Exhibition Center, Italy.
From Haikou Hainan to Milan, Italy, Poly has brought expert teams and business backbone to participate in the event. Poly has fully demonstrated its strong comprehensive strength and international strategic layout through numerous dimensions such as rich scientific and technological innovation research and development results, strict quality assurance system and firm international registration advantages, which has attracted many customers for in-depth exchanges and cooperation.
From October 8th to 10th, the grand 35th CPhi Milan 2024 (World Pharmaceutical Raw Materials Exhibition) was grandly opened at the Milan Exhibition Center, Italy, with more than 16,000 square meters of pharmaceutical raw materials field event attracting more than 2,400 exhibitors. A total of 62,000 pharmaceutical business owners, raw material suppliers, scientific research institutions and industry experts from 180 countries and regions gathered at the event, which is an annual event for the pharmaceutical API industry around the world, and has built a good platform to appreciate the most cutting-edge technologies and products of pharmaceutical science and technology and international trade cooperation.
Mr. Zhou, Deputy general manager of Hainan Poly Pharm. Group, Ms. Chen, Sales Director of the United States, Mr. Li, Sales Director of Europe, and Ms. Yuan, Director of Emerging Markets, discussed pharmaceutical cooperation with customers at the pharmaceutical exhibition.
Dr. Nie, general manager of Anhui Poly Pharm. and Ms. Xue, Sales Director, brought the raw material sales team to discuss the cooperation of APIs with customers at site.


Source: Sullivan's Blue Book on the Status and Trend of China's Biomedicine Development in 2024
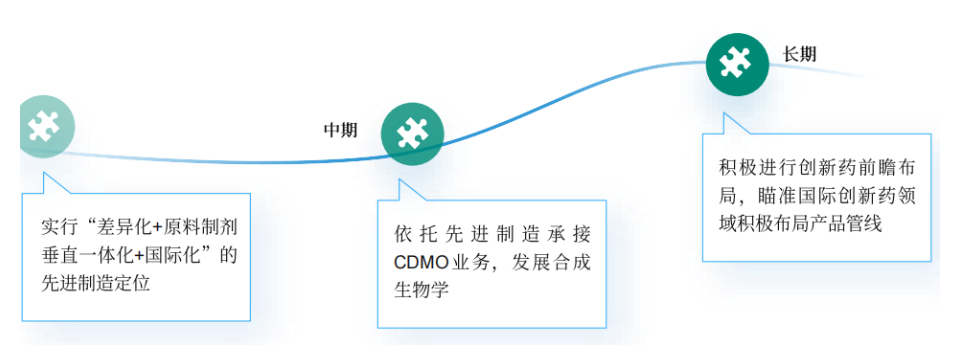
From the perspective of the company's development strategy, from the initial implementation of the advanced manufacturing positioning of "differentiation + vertical integration of raw materials and preparations + internationalization", a strategic system of key intermediates, APIs and preparations integration has been formed. Through independent production of raw materials and intermediates, Poly Pharm. has effectively achieved cost control and supply chain stability, and resource integration has realized a comprehensive cut-through of the pharmaceutical industry chain.
In the medium-term plan, it relies on advanced manufacturing to undertake CDMO business and develop synthetic biology. While expanding the global market of self-developed high-end preparations, Poly Pharm. enters the CDMO market with its own differentiated resource advantages. Gradually establish the brand influence of CDMO market at home and abroad, support global submission, raw materials + preparations + clinical one-stop service, advantages and characteristics of sterile injections, and rich dosage forms satisfy the demands of market scarcity. Horizontally expand the field of synthetic biology, focusing on the biosynthetic development of important raw materials for health care products, functional food and cosmetics, with prospective planning to achieve microbial fermentation production of multiple raw materials, complete industrial transformation, and have cost competitive advantages.
The long-term strategy actively carries out the forward-looking layout of innovative drugs, closely following the trend of new quality productivity, and innovation-driven, breakthrough innovations in boron neutron capture therapy (BNCT), innovative nanodrug (PLAT001), innovative dual-mode contrast agent (PL002) and other products promote the transformation and upgrading of the pharmaceutical industry and high-quality development.
Source: Sullivan's Blue Book on the Status and Trend of China's Biomedicine Development in 2024
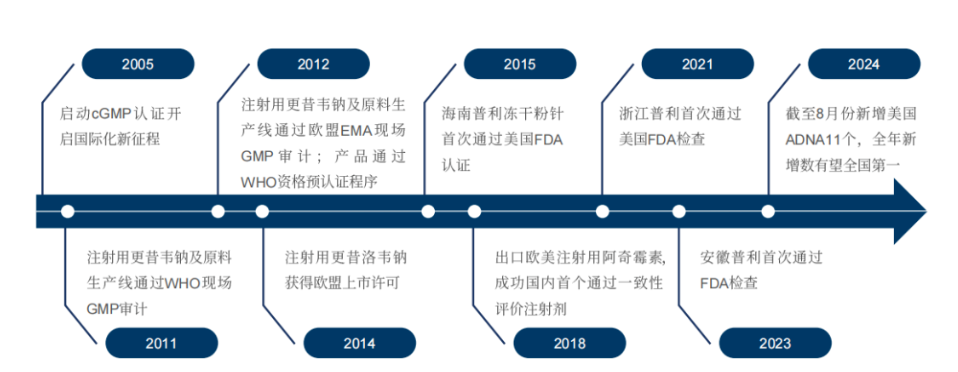
In 2005, Poly Pharm. established the strategy of simultaneous international and domestic development, focusing on the internationalization of injectables. In 2006, the company reconstructed the injection workshop in accordance with FDA and EU GMP. Since then, it has successfully passed the GMP inspection by WHO, EU and FDA for the first time in 2011, 2012 and 2015, respectively. Since the implementation of the internationalization strategy, up to now, 43 drugs have received a total of 174 international marketing approvals, including 36 ADNAs in the United States, of which 13 US ANDAs have been obtained by September 2024. The international markets that are being developed involve more than 60 countries and regions, including the United States, the European Union, the United Kingdom and many countries in the Middle East, Southeast Asia and along the Belt and Road, which has formed an international pattern of made in China and global sales.
■“Research”
①Strong R & D strength and rich registration experience
The company has a professional technical team of more than 500 persons, providing technology amplification and transformation services from laboratory trials to large-scale production. The team includes full-time registrants with an international perspective to provide strong support for the successful registration of the project
②The patent pool and product line reserve are rich, bringing potential growth points at home and abroad
Up to now, the company has obtained more than 110 patented technologies, including more than 90 invention patents, more than 340 product approval numbers, 174 overseas preparation production approvals in Europe and the United States, more than 80 approval and record document numbers for raw material production, and 14 approval and record document numbers for pharmaceutical excipients production. Through years of accumulation of international registration, it has formed a strategic arrangement of simultaneous registration at home and abroad for projects under development. At present, nearly 100 varieties are under development, involving the first generic drugs + patent challenge generic drugs, improved new drugs 505b(2) and new solid compound drugs.
■“Production”
①Build standardized and large-scale high-end production capacity
At present, the company has three internationalized production bases, that is, Hainan Poly, Zhejiang Poly and Anhui Poly, and more than 10 production lines have passed the certification of Europe and the United States, of which 8 workshops have passed the certification of the US FDA. At present, the dosage forms covered by the production line include injections (freeze-dried powder for injection, injection solution), tablets, capsules, dry suspension, oral solution, topical ointment, eye drops, pre-filled needles, APIs, etc..
②“The order-based production mode of "set production by sales"
Market demand-oriented and the implementation of supply chain whole process management. At the same time, the company continues the digitized, automatic and intelligent construction, and builds smart factories. Promote the construction of WMS, LIMS and other systems, and improve the operational efficiency and the competitiveness of the production system to better meet the flexible sales market demands
■“Marketing”
①Based on Hainan Free Trade Port, build a global sales network
Make full use of the Hainan Free Trade Port policy, improve product competitiveness, and further expand the international market; the company has been approved as the second batch of Hainan Free Trade Port "first-line liberalization, second-line management" import and export policy pilot enterprises, and enjoys the "processing value-added 30% and duty-free for export to the mainland" and other 8 specific policies, which have brought broader opportunities for international trade cooperation.
②Actively participate in local procurement and expand sales routes
Through in-depth cooperation with local purchasers, Poly Pharm. has increased market revenue. Up to now, it has reached a 7-year strategic cooperation with the major purchasing GPO private brands in the United States on ganciclovir for injection.
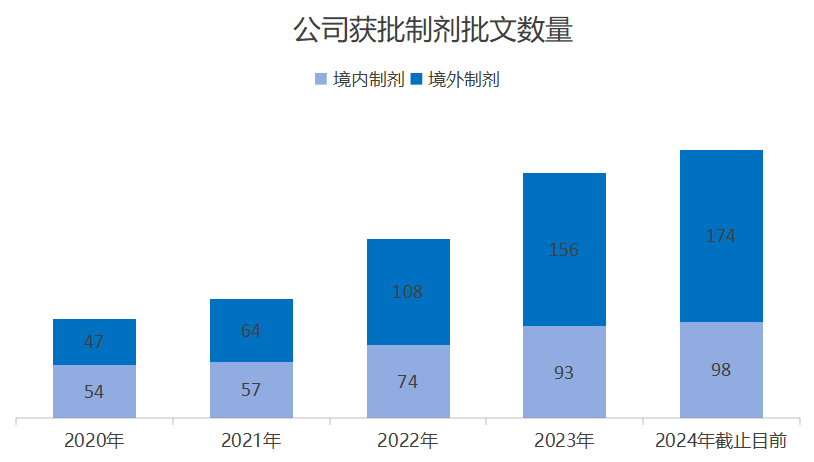
From 2020 to 2023, the company's preparation production approvals have shown a rapid growth trend, with the number of domestic approvals increasing from 54 to 93, and the number of overseas approvals in Europe and the United States increasing from 47 to 156. Up to now, Poly Pharm. has 43 drugs with a total of 174 international pharmaceutical marketing licenses, including 98 domestic approvals, and is developing the international markets involving more than 60 countries and regions, including the United States, the European Union, the United Kingdom and many countries in the Middle East, Southeast Asia and along the Belt and Road, which has formed an international pattern of made in China and global sales.
With the increase in the export number of China's generic drugs, the international market's recognition on the quality of China's generic drugs continues to strengthen, and under the scale effect, China's pharmaceutical industry will usher in a leap and transformation. As a landmark export enterprise, Poly Pharm. will enrich the generic drug production line, lay out the generic drugs with high technical barriers and enhance the development ability of the first generic drug, and inject a steady stream of new vitality into the development of the global pharmaceutical industry.
Here, we would like to extend our deepest gratitude to the new and old customers who have been supporting and trusting Poly Pharm. for a long time. It is your continuous support that has become an inexhaustible driving force for our growth and development. Poly Pharm. promises that it will continue to do its best to bring more customers a more professional and intimate service experience.
