

From Milan to the ancient capital Xi'an, after participating in the 35th CPHI Milan 2024 (World Pharmaceutical API Exhibition) held at the Milan Exhibition Center in Italy, Poly witnessed the grand opening of the 91st CPHI/Intermediates/Packaging/Equipment Fair from October 16-18, 2024.
Anhui Poly Pharm. (hereinafter as Anhui Poly) has brought expert teams and business backbone to participate in the event. With its deep cultivation and meticulous work in the field of technological innovation products, continuous optimization and improvement of quality system, and strategic layout of international registration, Poly has forged a solid and strong comprehensive competitiveness. This series of achievements not only demonstrates the company's strong strength, but also attracts many elites in the industry like magnets to negotiate cooperation and seek a blueprint for development together.
From October 16th to 18th, the 91st China CPHI/Intermediates/Packaging/Equipment Fair (API China) was grandly opened at the Xi'an International Convention and Exhibition Center, attracting more than 1000 outstanding API companies and professionals from China. The exhibition aims to gather top domestic enterprises, cutting-edge technologies, and innovative products, and build a pharmaceutical platform that integrates exhibition, exchange, cooperation negotiation, and trend exploration.







From the initial implementation of the advanced manufacturing positioning of "differentiation + vertical integration of raw materials and preparations + internationalization", a strategic system of key intermediates, APIs and preparations integration has been formed. Through independent production of raw materials and intermediates, Poly Pharm. has effectively achieved cost control and supply chain stability, and resource integration has realized a comprehensive cut-through of the pharmaceutical industry chain.
Anhui Poly Pharm. Co., Ltd., a wholly-owned subsidiary, was established in 2019 and is committed to building a distinctive supplier of high-quality chemical synthesis, biomanufacturing APIs and services to the global pharmaceutical, healthcare, and skincare fields.
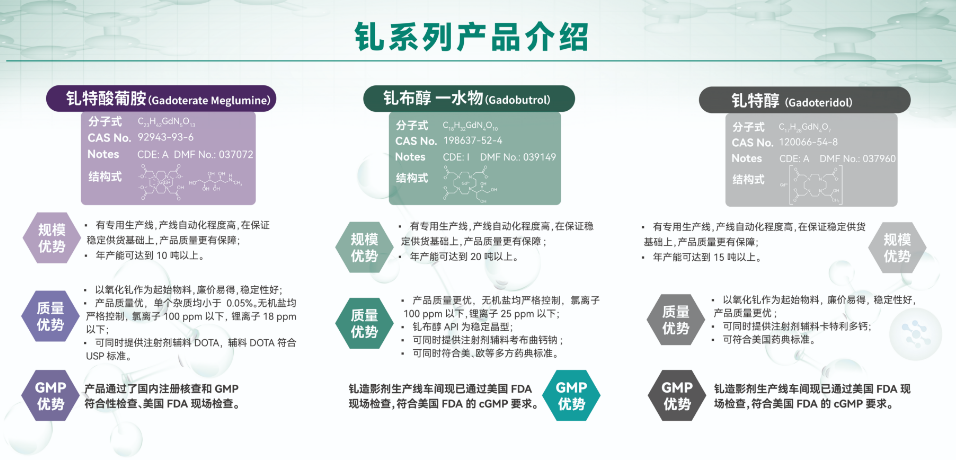
Through technological innovation, we have successfully completed the research and industrial transformation of a series of products including gadoterate meglumine, gadetoridol, gadobutrol, ioversol, iohexol, iopamidol, iodixanol, Ethiodized oil, etc., and have the ability to supply.
In addition, the company has also completed the research and development, production, and commercial conversion of a series of products including Magnesium Hydroxide, Sodium Nitroprusside, Dobutamine Hydrochloride, Tecovirimat, Brincidofovir, Voriconazole, Apremilast, Cyclophosphamide, Crisaborole, and Memantine Hydrochloride.
Products related to the 10th batch of national centralized procurement of drugs, including APIs such as Dobutamine Hydrochloride, Apremilast, and Gadoterate Meglumine, Anhui Poly will take the lead in implementing preferential supply policies. We sincerely invite and warmly welcome colleagues in the industry to inquire and explore new industry knowledge and cooperation opportunities together.
Poly Pharm. has carried out an integrated and forward-looking layout from APIs to formulations for gadoterate Meglumine, achieving resource integration of the entire product industry chain.
■ Preparation Area:After completing the technical research and development, the product has gradually submitted applications for market launch to multiple domestic and international markets.
We have obtained approval notices from the US Food and Drug Administration and drug registration approvals from the National Medical Products Administration in June 2024, and are qualified to be marketed and sold in China and the United States. The injection solution shares a production line with the US market, with the same quality standards and product quality, providing high-quality medication options for domestic patients.
■ API Area:Complete the registration/filing of APIs in Chinese and American markets
The US DMF number is 037072 (status: A; already bundled with preparation, FAL has been received)
①The gadoterate Meglumine API has passed technical review on DMF in the United States and received the First Adequate Letter (FAL) issued by the US Food and Drug Administration (FDA).
②The CN DMF number is Y20220000653 (status: A; already bundled with preparation), it has passed the domestic registration verification and GMP compliance inspection; And the workshop passed the FDA on-site inspection in 2023, and the production of API meets the cGMP requirements of the US FDA. The products are stable and continuously supplied to global customers.
■ The company has successfully developed the API Semaglutide through synthetic biology technology. This product is fermented with genetically engineered strains to obtain a peptide backbone, which is then obtained through a two-step chemical reaction. The purity of the product reaches over 98%. At present, the company has completed the process development and large-scale production of high-purity API.
■ At the same time, the company has completed the laboratory, scale-up, and validation batches of production for the key excipient SNAC used in the oral formulation of semaglutide, and has applied for CDE and DMF approval in the United States.
■ Relying on the synthetic biology platform, the company also focuses on the biosynthetic development of important raw materials for health products, functional foods, and cosmetics. Using gene editing technologies such as CRISPR/Cas, the company rationally reconstructs the product synthesis pathway in various industrial production chassis cells such as Escherichia coli, yeast, and Corynebacterium glutamicum. Combined with metabolic pathway network regulation optimization, the company has achieved microbial fermentation production of multiple raw materials, including Ectoine, Salidroside, and NMN, completing industrial transformation and possessing cost competitive advantages.
The company is equipped with modern instruments and production equipment. At present, it has 3 production workshops, 357 sets of multi-functional equipment, including advanced equipment such as ultrafiltration, nanofiltration, lyophilization, centrifugation, three in one, DAC column, spray drying, automatic subcontracting, etc.
The company has built three API production workshops that comply with GMP standards in Europe, US, and China, including iodine contrast agent production line, gadolinium contrast agent production line, small variety production line, anti-tumor production line, ordinary production line, and biological fermentation production line. One tumor preparation workshop, 15 production lines, the company has established a strict cGMP quality system for high-end APIs in Europe and US, as well as a high standard EHS system to ensure product quality and provide sustainable high-quality services.
Here, we would like to extend our deepest gratitude to the new and old customers who have been supporting and trusting Poly Pharm. for a long time. It is your continuous support that has become an inexhaustible driving force for our growth and development. Poly Pharm. promises that it will continue to do its best to bring more customers a more professional and intimate service experience.
