
Anhui Poly Pharm. Co., Ltd. (hereinafter referred to as “Anhui Poly”), a holding subsidiary of Hainan Poly Pharm. Co., Ltd. (hereinafter referred to as “Poly Pharm.”), has recently received Certificate of Suitability (CEP) of gadobutrol API issued by European Directorate for the Quality of Medicines & HealthCare (EDQM).

The CEP certificate indicates that the Gadobutrol API meets the quality requirements of the European Pharmacopoeia and shows the recognition and affirmation of the quality of the API by the European regulatory market, bringing positive impact on expanding of international market by Anhui Poly.
In addition, the production workshop has passed the FDA on-site inspection in 2023, the API manufacturing is in compliance with FDA's cGMP requirements, and the products will be supplied to global customers stably and consistently in the future.
Gadolinium contrast agent is the most common paramagnetic contrast agent, introduced into the human body through intravenous injection. After injecting contrast agents for enhanced scanning, the image can more clearly display the edges or adjacent tissues, internal structures, blood supply, and other conditions of the lesion, which is conducive to lesion detection and helps with disease diagnosis.
According to statistics, since gadolinium contrast agents were first approved for human clinical diagnosis and treatment, the application rate of gadolinium contrast agents in magnetic resonance imaging examinations has reached 35%, and more than 400 million gadolinium contrast agent injections have been performed worldwide.
Gadobutrol is a large cyclic non-ionic gadolinium contrast agent that combines high concentration and high relaxation rate. It provides clearer imaging and combines the effectiveness and safety of contrast-enhanced imaging. It is suitable for whole-body examinations of all ages (including full-term newborns), including patients with renal dysfunction.
According to the IMS database, except for a slight decrease caused by force majeure due to global public health events in 2020, the global sales of gadobutrol injection showed steady growth from 2018 to 2023 and exceeded 400 million US market demand in 2023.
The global consumption of APIs is also steadily increasing, with a market volume of 72 tons by 2023. The year-on-year growth rate is higher than the sales revenue, reflecting a faster increase in market demand for APIs.
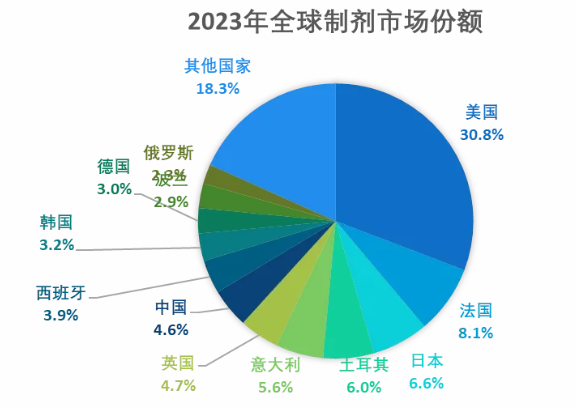
In 2023, the proportion of the global preparation market is dominated by the European and American markets. The top five sales will be the United States, France, Japan, Turkey and Italy, accounting for about 57% in total.
From the perspective of global consumption of APIs in 2023, the top five in market consumption are the United States, Turkey, China, Poland and Germany, accounting for 65% in total. Anhui Poly’s gadobutrol API has been qualified to enter major markets such as the United States, Turkey, China, Poland and Germany.

1)Simultaneously comply with multiple pharmacopoeia standards such as China, the United States, and Europe; The product quality is superior to the reference preparation, with strict control of inorganic salts, chloride ions below 100ppm, and lithium ions below 25ppm.
2)Gadobutrol API is a stable crystalline form with better stability
3)A dedicated production line with a high degree of automation, ensuring stable supply and an annual production capacity of over 20 tons.
4)Simultaneously provide excipients such as caclobutrol for finished injection.
5)The gadolinium contrast agent production line workshop has passed the on-site inspection of the US FDA and meets the cGMP requirements of the US FDA.
This API has been filed/registered in China and US markets
①The US DMF number is 039149 (status: A)
②The CN registration No. is Y20240000083 (Status: I)
③CEP No.: CEP 2024-082- Rev 00, in compliance with the quality requirements of EU.
④The obtaining of WC certificate entry qualification for Turkey market.
Meanwhile, registration in other markets is also in progress.
Anhui Poly would exert its product advantages, international registration ability (our registration team has completed registration of the product in many countries, and has a wealth of international registration experience), as well as internationalization manufacturing, quality control system and mature international market development ability, to provide all-round and in-depth cooperation with domestic and international drug product customers who are interested in market development of cyclophosphamide, creating a better future.
Anhui Poly Pharm., located in Anqing National High-tech Industrial Development Zone, is a professional supplier of active ingredients, dedicated to providing chemically synthesized and biosynthesized high-quality specialty ingredients to the global pharmaceutical, healthcare, and skin care sectors
At present, Anhui Poly has 10 production lines and 357 multifunctional equipment which meet the GMP standards of EU, US and China.
Relying on strong R&D capabilities and technological transformation capabilities, Anhui Poly has successfully completed the R&D and production of contrast agents (including Gadoterate Meglumine, Gadoteridol, Gadobutrol, Iomeprol, Iopromide, Ioversol, Iopamidol, Iodixanol), synthetic biology (including Semaglutide, Ursodeoxycholic Acid, Ectoin & Salidroside), anti-tumor substance (BPA, Cyclophosphamide), other APIs (Dobutamine hydrochloride, Sodium nitroprusside, Magnesium Hydroxide, Apremilast, Crisaborole, Voriconazole, glycopyrronium bromide , Memantine Hydrochloride, Tecovirimat, Acyclovir sodium, Ganciclovir sodium, Bumetanid, Fluconazole, Selenium sulfide, Alloporinol), and other pharmaceutical excipients (DOTA, SNAC, Betadex Sulfobutyl Ether Sodium, Calteridol Calcium, Calcobutrol).
