
Anhui Poly recently received the NMPA issued the approval of the marketing application of chemical APIs, the involved product is magnesium hydroxide API. The approval of this marketing application indicates that Anhui Poly magnesium hydroxide API products can be used in various downstream preparation dosage forms, the company will continue to maintain stable product quality and production capacity to meet the market demand, to ensure that the company's normal production and operation.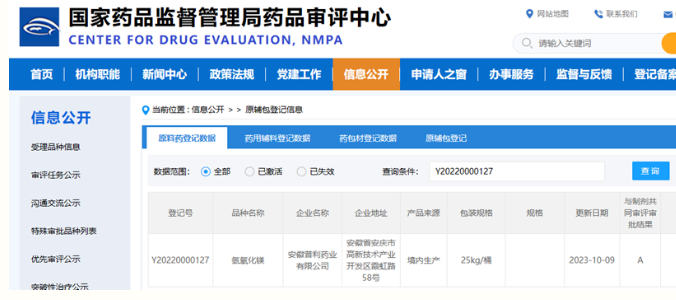
Magnesium hydroxide belongs to the antacid class of drugs and has the effect of neutralizing stomach acid and relieving diarrhea. In addition, as a food additive, it has a magnesium tonic effect, and to a certain extent, anti-anxiety effects.The downstream application of API is used in various pharmaceutical preparations such as :Famotidine Calcium Carbonated and Magnesium Hydroxide Chewable Tablets, Heavy Magnesium Carbonate and Aspirin Tablets, Aluminum and Magnesium Chewable Tablets, Aluminum and Magnesium Suspension, Aluminum and Magnesium Dimethicone Chewable Tablets, and Lansoprazole Enteric-coated tablets, etc.; it can also be used in special medical food (enteral nutritional solution), and magnesium hydroxide contributes magnesium ions when used in the products of enteric suspensions. In addition, in the food field, magnesium hydroxide is also widely used in health care products, food additives, neutralizing agents, inhibitors.
According to the IMS database, the global size of magnesium hydroxide pharmaceuticals reached $100 million in 2022, and the global consumption of APIs reached 1,276 tons.
1、Quality Advantage:
1)Anhui Poly's magnesium hydroxide is prepared by ammonia synthesis method, which effectively removes foreign matter through precision filters; whereas magnesium hydroxide from mineral source adopts high-temperature hydrolysis process of mineral magnesium oxide, which makes it difficult to remove impurities and is seen to have a lot of foreign matter;
2)Anhui Poly's magnesium hydroxide in the production process through the ammonia and metal ions to form compatibilizers to remove the metal elements, effectively reduce the metal ion residue, the product metal ion residue is low, especially class I and 2A elemental impurities are far below the limit requirements; and other mineral sources of magnesium hydroxide, the impurity does not have an effective means of removing the elemental impurities, elemental impurities of the residue of the risk is high, there is a potential security risk;
3)Anhui Poly's magnesium hydroxide effectively control the rate of crystal precipitation, the production of magnesium hydroxide particle size is larger, the conventional particle size of about 100 microns, through the crushing can meet the needs of D (90) 2 ~ 100 microns of all particle size;
4)Anhui Poly's magnesium hydroxide quality all meets the standards of ChP, JP, EP, USP, KP.
2、Scale Advantage:
1)There is a dedicated production line for stable supply.
2)Capacity up to 200 tons per year
3、Registration Regulations Advantage:
This product layout is registered globally and has been filed/registered as API in China, US, EU and other markets.
USA: US DMF No. 036567 (Status: A), China: CN Registration No. Y20220000127 (Status: A), EU: CEP certificate issued by EDQM, Certificate No. R0-CEP 2022-010-Rev 00, passed the domestic GMP Compliance check, and obtained WC certificate.
Meanwhile, registration in other markets such as Korea and Japan is in progress.
Anhui Poly Pharm. is a professional supplier of active ingredients, dedicated to providing chemically synthetic, biosynthetic and high-quality raw materials and services in fields of pharmaceutical industry, health care and skin care worldwide.Relying on strong R & D capability and technology conversion ability, Anhui Poly Pharm. Co., Ltd. has successfully completed the process development and quality research of magnesium hydroxide API, and successfully realized commercial production in the international high-end API production base in Anqing —— the current product status to A, can be associated with downstream preparations, stable and sustained service to global customers. Currently, the product status has been changed to A, which can be associated with downstream preparations and serve global customers stably and continuously.Inorganic APIs have simple structure and clear mechanism of action, which has a broad market prospect. Magnesium hydroxide is one of the inorganic API varieties in the layout of Poly Pharm., and its approval marks another solid step for Poly Pharm. in the inorganic API market.
