
Poly Pharm. has successfully developed Ursodeoxycholic Acid (UDCA) through synthetic biology technology. The product is obtained through genetically engineered strains of bacteria using a multi-enzyme co-catalytic method for specific substrates, and the purity of the product reaches more than 99%.In the future, ursodeoxycholic acid will be registered in China, the United States, Europe, Japan, Korea and other markets.
Ursodeoxycholic acid is a hydrophilic dihydroxybile acid extracted from bear bile and is a unique component of bear bile. Ursodeoxycholic acid (CDCA) is synthesized in small amounts by metabolic reactions in the intestine and accounts for about 3% of bile acids. Ursodeoxycholic acid can increase the secretion of bile, inhibit the cytotoxicity of hydrophobic bile acids, protect the bile duct cells and hepatocytes from toxic bile damage, and improve the morbidity and mortality of patients; meanwhile, it is able to improve the liver enzyme levels of primary biliary cirrhosis (PBC) patients after liver transplantation by improving liver enzymes levels, delaying hepatic histology, and thus reducing the recurrence rate of the disease as well as prolonging the survival period.In 1997, the U.S. FDA approved UDCA as the only first-line therapeutic agent for the treatment of PBC.
Ursodeoxycholic acid (UDCA) was originally extracted from bears, and in view of its high medical value, the development of synthetic ursodeoxycholic acid (UDCA) by chemical and biological methods to meet the urgent clinical needs has become a hotspot of drug research in this field. The chemical synthesis method is usually based on the easy-to-obtain animal cholic acid (CA) as the API, and UDCA is synthesized by redox. although this method is simple in operation, cheap in reagents, and mature in process, the chemical toxic reagents and chiral difficulties have become the bottleneck of the industrial technology transformation. The biosynthesis method, on the other hand, utilizes biological enzymes to catalyze the synthesis of UDCA, which has the advantages of greenness, simplicity and high efficiency, and has become the mainstream of industrial production of UDCA.
UDCA improves bile cholesterol saturation and slows disease progression and is currently the global consensus first-line treatment for primary biliary cholangitis (once known as primary biliary cirrhosis, PBC).In addition to PBC, UDCA is widely used clinically for bile reflux gastritis, other cholangitis, intrahepatic cholestasis in pregnancy, and hepatoprotection in the context of a variety of diseases. Treatment of hepatic and biliary diseases usually involves correcting the causative factors while improving key markers, reversing the course of the disease, and taking full advantage of the compensatory nature of the liver to maximize the quality of survival during the course of the disease.In December 2022, researchers from the University of Cambridge published a research paper entitled: FXR inhibition may protect from SARS-CoV-2 infection by reducing ACE2 in Nature, a leading international academic journal. The study showed that UDCA was able to prevent infection by the new coronavirus and may have the ability to prevent infection by future mutant strains of the new coronavirus.
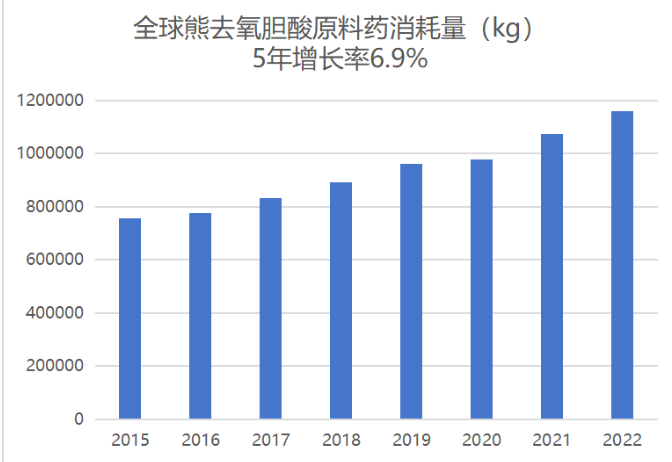
IMS data shows that the global ursodeoxycholic acid sales volume of 639 million tablets/capsules and API consumption of 1,158 tons in 2023 is in a long-term growth trend, with a 5-year CAGR of 6.9%.
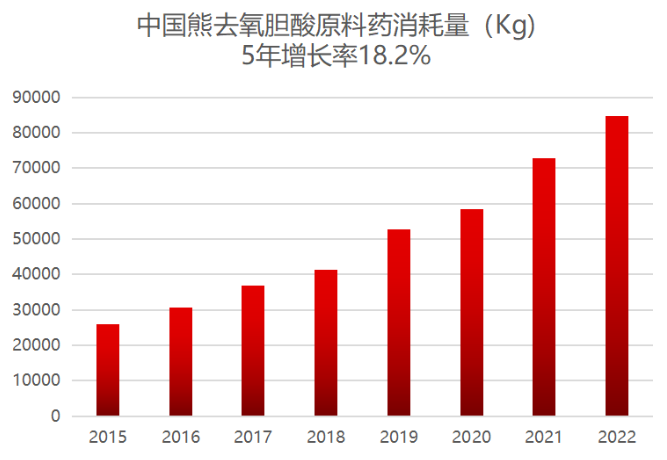
In 2022, China ursodeoxycholic acid API consumption of about 84 tons, for the global consumption of 7.3%, compared with China accounted for 18% of the world's population there is still a large space for growth, is in a period of rapid growth, 5-year compound growth rate of 18.2%.
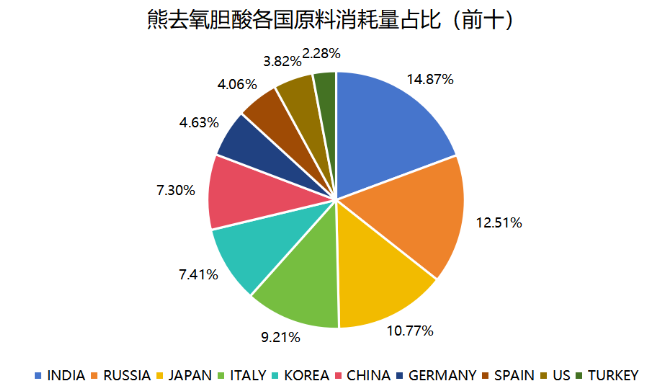
IMS data shows that the consumption of ursodeoxycholic acid API in Japan in 2022 is about 125 tons, which is 10.8% of the global consumption; the consumption of ursodeoxycholic acid API in South Korea is about 86 tons, which is 7.4% of the global consumption; and the consumption of Russia, Italy, Germany, and Spain among the European countries is in the top ten globally, which is about 346 tons in the whole Europe. The consumption of the United States is also in the global top ten.
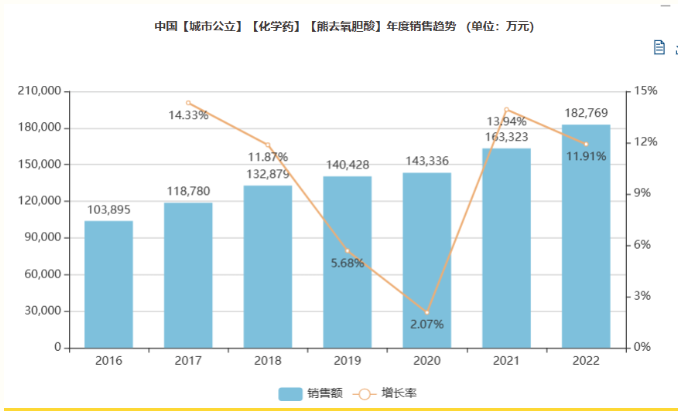
In fact, ursodeoxycholic acid tablets/capsules have grown to be a large 2.8 billion variety in China. Menet data shows that the sales of ursodeoxycholic acid tablets/capsules have reached RMB 2.56 billion in public hospitals and RMB 230 million in retail pharmacies in 2022.2022 Ursodeoxycholic acid tablets/capsules were included in the eighth round of the centralized drug-procurement program, with an agreed purchase volume of 127,475,378,000 tablets/capsules in the first year. With the increase of national medical anti-corruption efforts, the centralized drug-procurement varieties and their APIs have significant policy benefits.
Ursodeoxycholic Acid, as one of the R&D varieties of Poly Pharm.'s synthetic biology R&D platform, we have self-developed genetically engineered bacterial strains, and we have realized the reduction of production cost through the directed modification of multi-enzyme catalytic technology and have completed the development of scaling-up process and product quality research, and we can obtain kilograms of high-purity samples.Poly Pharm.'s biological R&D platform focuses on synthetic biology technology, realizes the biomanufacturing of high value-added active products and important pharmaceutical intermediates, and empowers original research and innovation in the biopharmaceutical industry.
In the future, ursodeoxycholic acid will be registered in China, the United States, Europe, Japan, South Korea and other markets to steadily and continuously serve global customers. Welcome to explore the global market of ursodeoxycholic acid capsules & tablets.
