
Anhui Poly Pharm. Co., Ltd. (hereinafter referred to as "Anhui Poly"), a subsidiary of Hainan Poly Pharm. Co., Ltd. (hereinafter referred to as "Poly Pharm."), has recently received the Notice of Approval about application for marketing of cyclophosphamide chemical raw material issued by the National Medicinal Product Administration (hereinafter referred to as "NMPA") (Notice number: 2024YS00429).
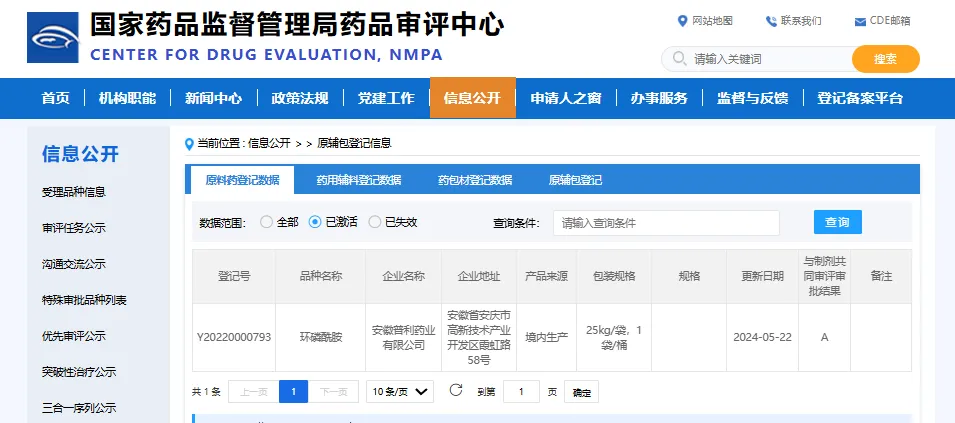
The marketing application has been approved, indicating that Anhui Poly’s cyclophosphamide API has realized commercial production, registration application has been successfully transferred to A, can be associated with domestic downstream preparations, and the workshop passed the FDA on-site inspection in 2023; drug production meets the cGMP requirements of the US FDA, and the future product will be stable and continue to serve global customers.
Basic Information of the Product:
1) Product name: cyclophosphamide
2) Dosage form: API
3) Registration No.: Y20220000793
4) How supplied: 25kg/drum
5) Holder: Anhui Poly Pharm. Co., Ltd.
Information of the Preparation:
Cyclophosphamide is a cytotoxic drug of alkylating agents, which can interfere with the DNA and RNA functions, cross-bind with DNA, and inhibit DNA synthesis. Preparation forms include tablets, capsules and injections, etc. The drug has a wide anti-tumor spectrum and wide clinical application, and has certain curative effects on malignant lymphoma, acute or chronic lymphocyte and leukemia.
Anhui Poly can supply up to 10 tons of raw materials for different preparation forms of cyclophosphamide (powder for injection, tablet, capsule, injection). At the same time, it is a cytotoxic production line, which has the production capacity for various types of cytotoxic products.
Product Advantages:
1、Quality Advantages
1) The product quality is comparable to the reference preparation, the microbial limit is strictly controlled, the total aerobic bacteria is less than 10cfu/g, bacterial endotoxins is no more than 0.025EU/mg;
2) It is manufactured on the dedicated line of the cytotoxic workshop, the annual supply can reach 10t, the production line has a highly automatic, and the product quality is better guaranteed on the basis of ensuring stable supply.
2、Regulatory Advantages
This product has been filed/registered as an API in China and the United States,
1) Where US DMF number is 037154 (Status: A; and has been cited by preparation, FAL has been received); The cyclophosphamide API has passed the technical review in the US DMF, and received the First Adequate Letter (FAL) of cyclophosphamide API issued by the US Food and Drug Administration (FDA). The workshop passed the FDA on-site inspection in 2023, and the drug production meets the cGMP requirements of the US FDA;
2) CN registration number is Y20220000793 (Status: A), and has passed the domestic registration verification and GMP compliance inspection.
3) It is expected to receive the CEP certificate in September 2024, At the same time, the company is also actively promoting the registration in other markets.
Anhui Poly:
Anhui Poly Pharm. Co., Ltd., founded in 2018, is located in Anqing National High-tech Industrial Development Zone. It is a specialist supplier of active raw materials, providing high quality chemosynthetic and biosynthetic specialty raw materials to the pharmaceutical, healthcare and skincare sectors worldwide.At present, the company has 3 API production workshops, 10 production lines and 357 multi-functional equipment that meet the GMP standards of Europe, the United States and China.Relying on strong research and development capabilities and technology transformation capabilities, the company has completed the commercial development and production of contrast agents (Gadoterate Meglumine, Gadoteridol, Gadobutrol, Iomeprol, Iopromide, Ioversol, Iopamidol, Iodixanol), synthetic biology (ectoin, salidroside), antitumor (cyclophosphamide), other API (Magnesium hydroxide, Apremilast, Crisaborole, Voriconazole, Memantine hydrochloride), and other pharmaceutical excipients (DOTA), SBE-β-CD, Calteridol calcium, Calcobutrol).
Cyclophosphamide is one of the high-end API varieties developed by Poly, having broad market prospects. The approval of this product marks another solid step for Poly Pharm. in this field and has a positive impact on the company's market expansion.
Welcome for enquiry and product supply for domestic and global clients; Look forward to CMO services in the anti-tumor workshop with GMP compliance.
