
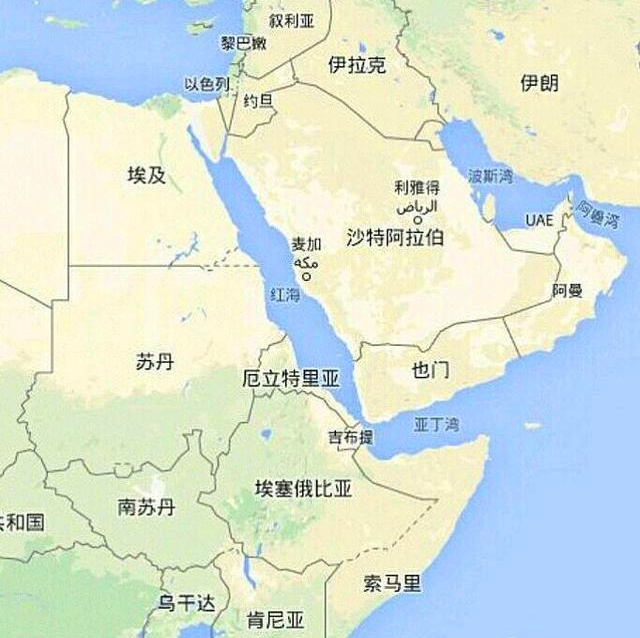
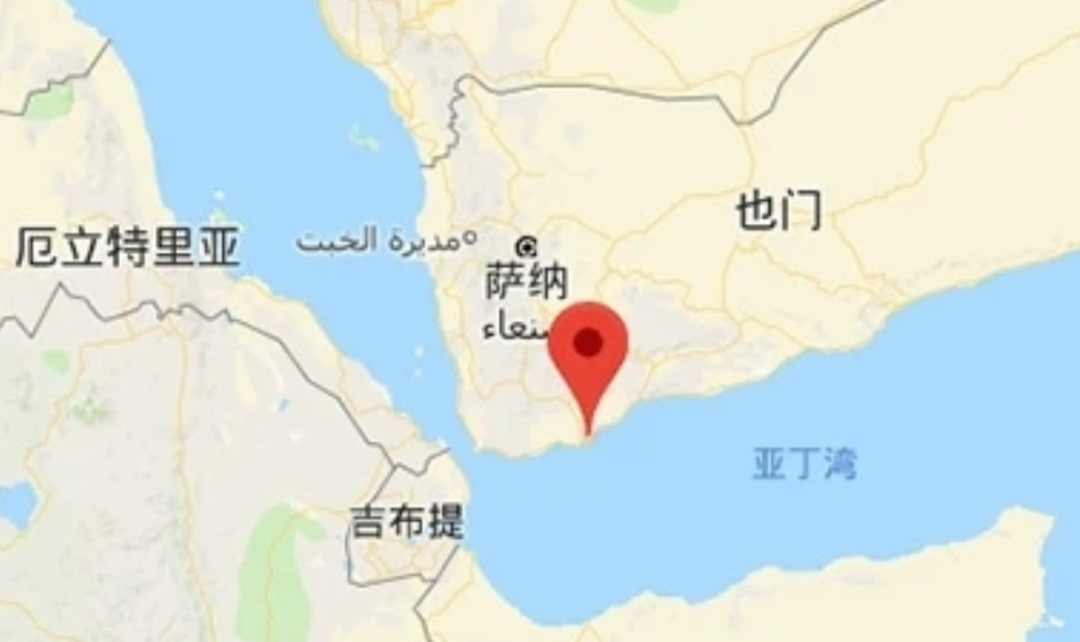
There have been clashes between Israel and the Houthis in recent days; On July 3, 2024, CEO of the Port of Eilat: The port is completely closed and there has been no activity at the port for eight months.
The port of Eilat is located at the top of the Gulf of Aqaba in the Red Sea and is Israel’s only port on the Red Sea. The port of Eilat was opened in 1947 and is now mainly used for trade between Israel and the Far East.
Dead Sea Bromine Co., Ltd is a subsidiary of Israel Chemicals Ltd. (ICL), which occupies the main market share of magnesium hydroxide in the world, and the main supply markets are in the Far East (Korea 500 tons, Japan 500 tons, Thailand 500 tons, etc.). The closure of the port of Eilat directly limits Dead Sea Bromine’s global supply of magnesium hydroxide.
At present, tensions in the Middle East are escalating, and geopolitical conflicts may further spread. In order to ensure the stable supply of magnesium hydroxide to global market, Anhui Poly is willing to exert its geographical and product advantages, international registration ability (our registration team has completed registration of the product in many countries, and has a wealth of international registration experience), as well as internationalization manufacturing, quality control system and mature international market development ability, to provide all-round and in-depth cooperation with domestic and international pharmaceutical customers who are interested in market development of magnesium hydroxide, creating a better future.
In the pharmaceutical field, magnesium hydroxide is in the category of antacids, which can neutralize gastric acid and relieve diarrhea. The downstream of API is used in various drug products such as Famotidine, Calcium Carbonate and Magnesium Hydroxide Chewable Tablet, Aluminum, Magnesium and Aspirin Tablet, Aluminum and Magnesium Chewable Tablet, Aluminum and Magnesium Suspension, Alumina, Magnesia and Dimethicone Chewable Tablet and Lansoprazole Enteric-Coated Tablet. It is estimated that the global consumption of API for various dosage forms of magnesium hydroxide would reach more than 2,000 tons.
Magnesium hydroxide can also be used in Food for Special Medical Purpose (Enteral Nutritional Suspension), where magnesium hydroxide contributes magnesium ions. In addition, in the field of food, magnesium hydroxide also has the effect of supplementing magnesium, and is widely used in health care products, food additives, neutralizing agents, inhibitors, etc.
1)Anhui Poly’s magnesium hydroxide is prepared by ammonia synthesis method, and foreign matter is effectively removed by precision filter; For magnesium hydroxide from mineral sources, the high temperature hydrolysis process of mineral magnesium oxide is adopted, but the impurities are difficult to remove, and there are many foreign matters.
2)For Anhui Poly’s magnesium hydroxide, metal elements is removed through the formation of complexes of ammonia and metal ions in the production process, effectively reducing metal ion residues; The metal ion residues of the product are low, especially the impurities of Class I and 2A elements are far below the limit requirements; However, magnesium hydroxide from mineral sources does not have an effective means to remove impurities, and the residue risk of elemental impurities is high, which has potential safety risks.
3)For Anhui Poly’s magnesium hydroxide, the crystallization speed could be effectively controlled, and the obtained magnesium hydroxide has a large particle size (conventional particle size is about 100 microns); through grinding, particle size requirements of D (90) 1-100 microns could be achieved, fulfilling the special requirements of particle size of different dosage forms such as dry suspension, capsules, tablets, etc.
4)The quality of Anhui Poly’s magnesium hydroxide conforms to ChP, JP, EP, USP and KP.
Anhui Poly plans to register this product in global markets, and the API has been filed/registered in China, United States and EU markets; US DMF number is 036567 (status: A):

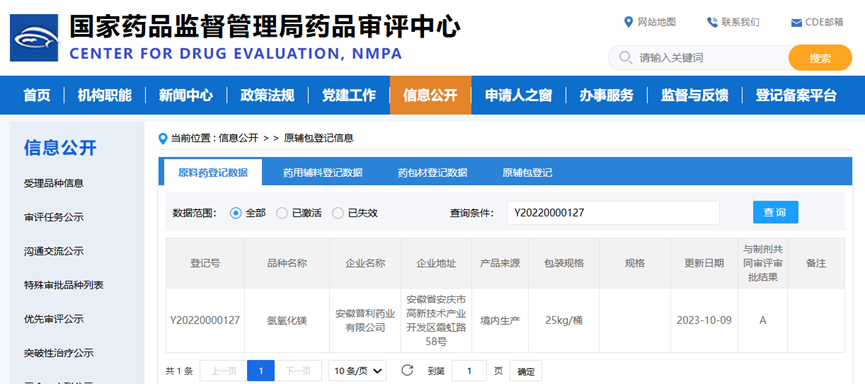
China: CN registration number is Y20220000127 (status: A)
EU: CEP certificate from EDQM obtained (certificate no.: R0-CEP 2022-010-Rev 00)

Domestic GMP compliance inspection passed and WC certificate obtained
Meanwhile, registration in other markets such as South Korea and Japan is also in progress.
At present, tensions in the Middle East are escalating, and geopolitical conflicts may further spread. The closure of the port of Eilat directly limits Dead Sea Bromine’s global supply of magnesium hydroxide. In order to ensure the stable supply of magnesium hydroxide to global market, Anhui Poly is willing to exert its geographical and product advantages, international registration ability (our registration team has completed registration of products in many countries, and has a wealth of international registration experience), as well as internationalization manufacturing, quality control system and mature international market development ability, to provide all-round and in-depth cooperation with domestic and international pharmaceutical customers who are interested in market development of magnesium hydroxide, creating a better future.
Anhui Poly Pharm., founded in 2018 and located in Anqing National High-tech Industrial Development Zone, is a professional supplier of active ingredients, dedicated to providing chemically synthesized and biosynthesized high-quality specialty ingredients and services to the global pharmaceutical, healthcare, and skin care sectors.
At present, Anhui Poly has 3 API production workshops, 10 production lines and 357 multifunctional equipment which meet the GMP standards of EU, US and China. Anhui Poly has strong and efficient R&D and production team, equipped with modern production facilities, and established a strict quality system complying with the requirements of Europe & US cGMP (for high-end APIs) and a high-standard EHS system. Anhui Poly is dedicated to providing not only high-quality APIs and intermediates, but also cooperative services including CDMO and process optimization.
Relying on strong R&D capabilities and technological transformation capabilities, Anhui Poly has successfully completed the R&D and production of contrast agents (including Gadoterate Meglumine, Gadoteridol, Gadobutrol, Iomeprol, Iopromide, Ioversol, Iopamidol, Iodixanol), Ectoin & Salidroside (by synthetic biology), anti-tumor substance (Cyclophosphamide), other APIs (Magnesium Hydroxide, Apremilast, Crisaborole, Voriconazole, Memantine Hydrochloride), and other pharmaceutical excipients (DOTA, Betadex Sulfobutyl Ether Sodium, Calteridol Calcium, Calcobutrol).
