
Director-General of World Health Organization Tedros declared that the monkeypox arose a “public health emergency of international concern” on August 14. This is the second time in just over two years that the WHO has declared that the monkeypox arose a “public health emergency of international concern”. More than 15,600 monkeypox cases have been reported so far this year, surpassing last year’s total, including 537 deaths, according to the WHO.
On August 13, the Africa Centres for Disease Control and Prevention declared that the monkeypox epidemic is an African public health emergency and called on African countries to take urgent action to avoid the continued spread of monkeypox on the continent. According to the Africa Centres for Disease Control and Prevention on August 8, at least 16 countries in Africa have been affected by monkeypox outbreaks. Africa has reported 160% more monkeypox cases this year compared to the same period in 2023.
Anhui Poly’s forward-looking layout of monkeypox disease prevention and control drugs, Brincidofovir and Tecovirimat APIs (self-developed and manufactured), facilitate the prevention and control of monkeypox epidemic.

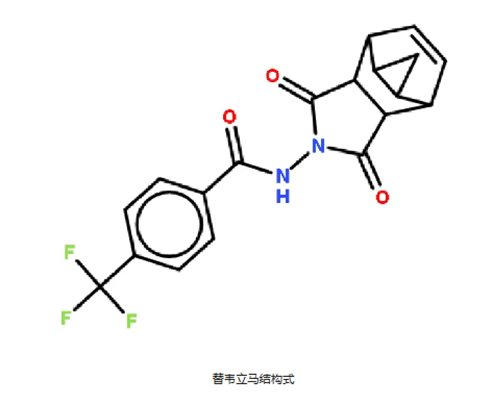
Tecovirimat (also known as ST-246) is a small molecule, broad-spectrum membrane protein inhibitor, by inhibiting the activity of the VP37 protein of the orthopxvirus, preventing the formation of the virus-specific envelope complex, preventing the export of infectious envelope virus particles, thereby inhibiting the transmission of the virus between cells and remote transmission. It has strong activity against orthopoxvirus such as variola virus, monkeypox virus, vaccinia virus and etc.
Developed by Siga Technologies, Tecovirimat was granted orphan drug status by the FDA for the treatment of poxvirus infections in 2010, and was approved for marketing in 2018 for the treatment of smallpox in adults and children with a weight of at least 13kg, and is the first approved anti-smallpox drug. In 2022, the European Medicines Agency approved it for the treatment of smallpox, monkeypox, vaccinia, and vaccinia complications following smallpox vaccination.
According to the information published on the website of the Centers for Disease Control and Prevention (CDC), Tecovirimat has been used in the off-label (compassionate use) form to treat patients infected with monkeypox virus since the outbreak of monkeypox disease in 2022, and has a positive effect on reducing the amount of virus in the body.
On September 15, 2023, the National Health Commission announced that according to the relevant provisions of the Law of the People's Republic of China on the Prevention and Control of Infectious Diseases, monkeypox would be included in Category B infectious diseases from September 20, 2023, and the prevention and control measures of Category B infectious diseases would be taken accordingly. The Monkeypox Prevention and Control Plan formulated and issued by the National Disease Control and Administration Center and the National Health Commission still focuses on prevention, and there is no mention of effective antiviral treatment plan.
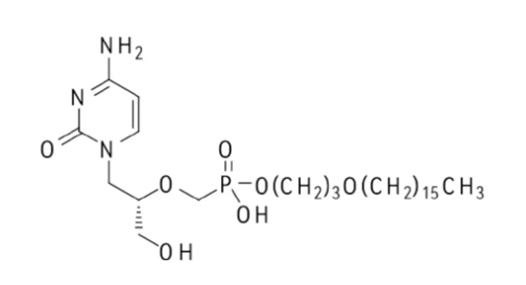
Brincidofovir (also known as CMX001) is an orally active lipophilic form of cidofovir (CDV), a prodrug of cidofovir that is designed to release cidofovir within cells. It has stronger in vivo and in vitro activity than CDV against certain herpes viruses, adenoviruses and orthopoxviruses, and is an antiviral drug used to treat cytomegalovirus, adenovirus, smallpox and Ebola virus infections. It is indicated for the treatment of human smallpox disease caused by smallpox virus in adult and child patients, including newborns. On June 04, 2021, the U.S. Food and Drug Administration approved Brincidofovir tablets and oral suspension for the treatment of smallpox.
Good product quality, with the purity of more than 99.9%; dedicated production lines, facilitating stable supply; annual output could reach more than 500kg;
Excellent product quality, with single impurity amount of less than 0.3%; dedicated production lines, available for customization for different clients; have provided API products for many domestic and foreign enterprises.
Anhui Poly plans to register this product in global markets, and the API has been filed/registered in China and the United States and other markets;
US DMF number is 038852 (status: A);

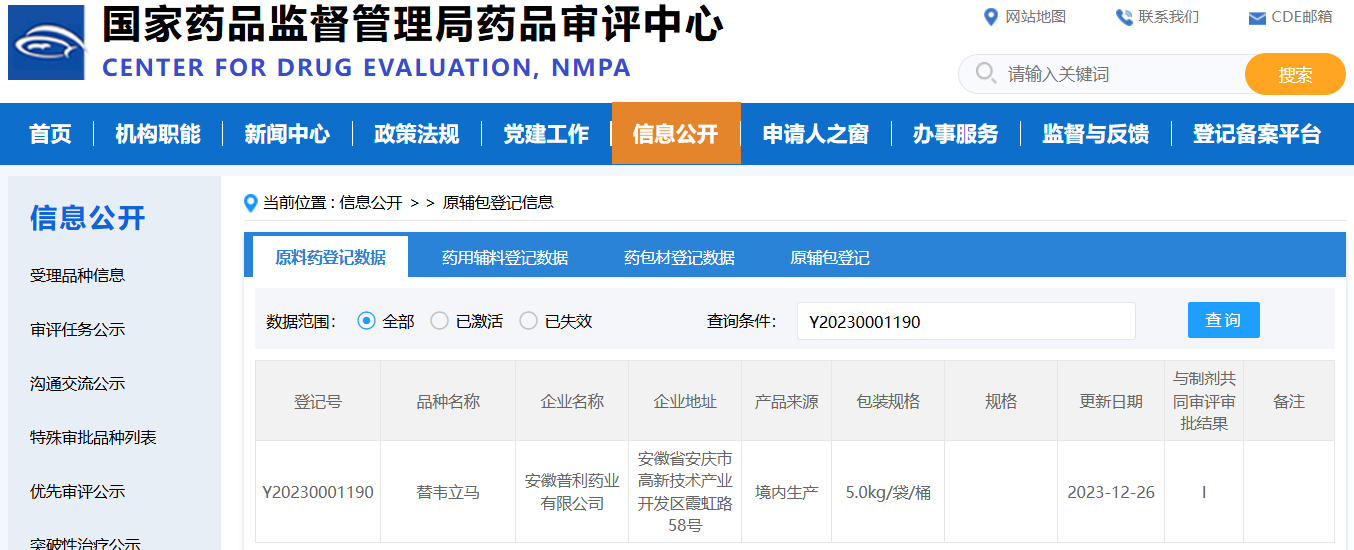
China: CN registration number is Y20230001190 (status: I);
At present, Tecovirimat has been marketed abroad with the dosage forms of injections, capsules, etc., and no drug products are marketed in China at present.
For injection, the production of scale-up batch is completed and that of registration batch is in progress. The registration application in domestic and overseas markets is already in progress.
Anhui Poly Pharm., a wholly-owned subsidiary of Hainan Poly, would give full play to the advantages of global registration and manufacturing internationalization, and use international high-end production capacity to help the research and development of monkeypox treatment drugs, and make joint efforts for the global monkeypox disease prevention and control.
Anhui Poly Pharm. is a professional supplier of active ingredients, dedicated to providing chemically synthesized and biosynthesized high-quality specialty ingredients and services to the global pharmaceutical, healthcare, and skin care sectors.
