
Romiplostim API is one of the strategic products in the high-end synthetic biology field of Anhui Poly’s layout, and the product has broad market prospects. We are willing to provide all-round and in-depth cooperation with domestic and international customers who are interested in developing this product to create a better future.

Romiplostim is a thrombopoietin (TPO) receptor agonist indicated for the treatment of primary chronic immune thrombocytopenia and is also a drug product for the treatment of radiation sickness.
Romiplostim, developed by Amgen, was approved by the U.S. Food and Drug Administration (FDA) on August 22, 2008, by the European Medicines Agency (EMA) on February 4, 2009, and by the Japan Pharmaceuticals and Medical Devices Agency (PMDA) on January 21, 2011. It is marketed by Amgen in the United States and Europe under the brand name Nplate (also marketed by Kyowa Kirin in Japan under the brand name Romiplate) and was approved for marketing in China on January 7, 2022.
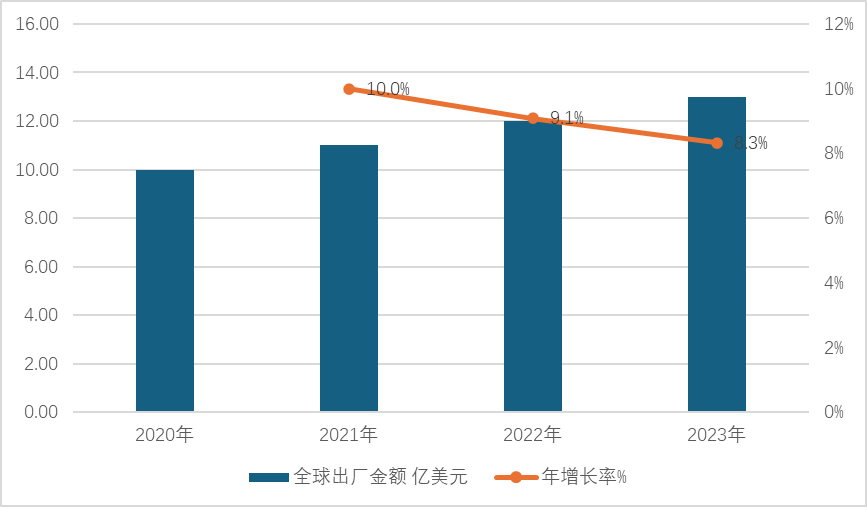
According to the IMS database, global sales of Romiplostim drug product has increased from $1 billion in 2020 to $1.3 billion in 2023, showing a steady growth trend.
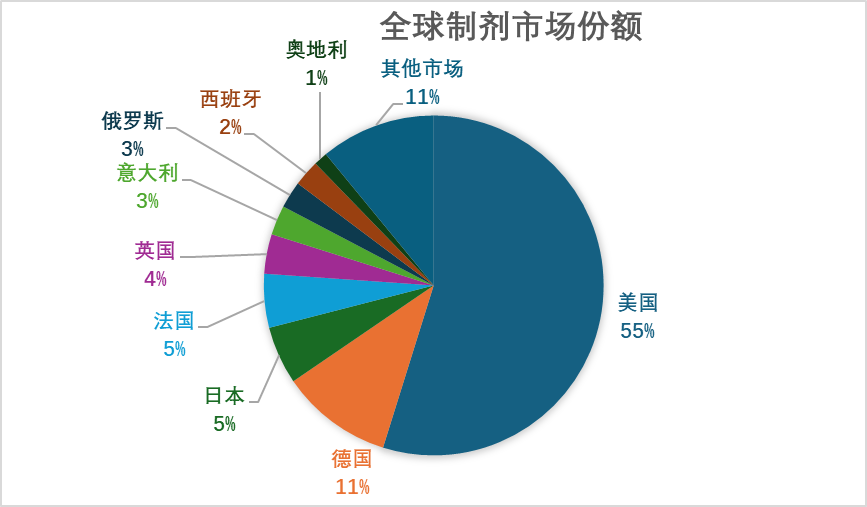
From the perspective of the global market proportion of drug product in 2023, the European and American markets play dominant role, and the main sales markets are the United States, Germany, Japan, France, the United Kingdom, Italy, Russia, Spain, etc., accounting for 89%.
Anhui Poly adopts the fermentation & semi-synthetic process, uses the self-developed genetic engineering strains to inoculate for fermentation culture, obtains the target protein fermentation product, and further obtains the Romiplostim API product that meets the requirements through the purification step. The unique structure sequence makes the product have advantages in property and product process quality.
Anhui Poly has an efficient and excellent R & D and international registration teams, with internationalization manufacturing, quality control system and mature international market development capabilities.
Adhering to the global internationalization strategy concept, Anhui Poly is willing to provide a full range of customized services for CDMO/CMO partners heading towards the international market, and is also willing to cooperate with international enterprises to create a better future.
Anhui Poly Pharm., located in Anqing National High-tech Industrial Development Zone, is a professional supplier of active ingredients, dedicated to providing chemically synthesized and biosynthesized high-quality specialty ingredients to the global pharmaceutical, healthcare, and skin care sectors.
At present, Anhui Poly has 3 API production workshops, 10 production lines and 357 multifunctional equipment which meet the GMP standards of EU, US and China.
Anhui Poly has a strong and efficient R & D and production teams, is equipped with modern production facilities, and has established strict European and American high-end API cGMP quality system and high standard EHS system, to provide customers with high-quality APIs and intermediates, as well as CDMO, process technology transformation and other services.
