
Recently, the pharmaceutical excipient betadex sulfobutyl ether sodium produced by Anhui Poly Pharm. Co., Ltd (Anhui Poly), a holding subsidiary of Hainan Poly Pharm. Co., Ltd (Poly Pharm), was approved in the joint review with drug products on the API, Excipient, and Packaging Material Platform of China’s NMPA Center for Drug Evaluation (CDE). This means that the product passed the associated review and was approved for use in marketed drug products. The product is stable and available for customers worldwide.
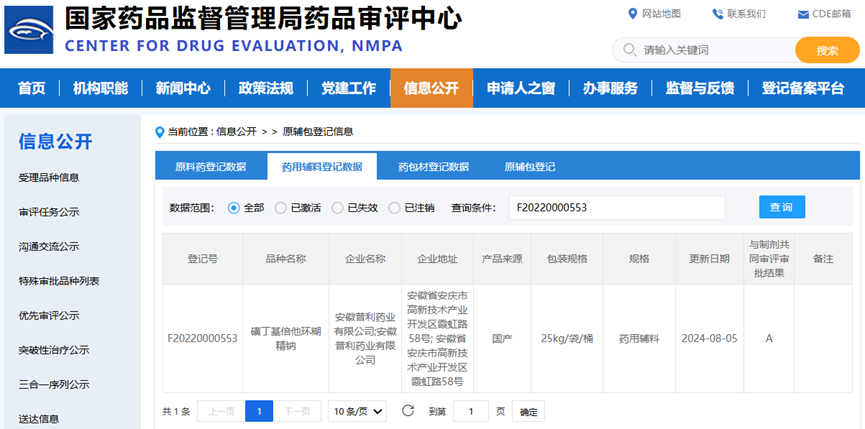
1) Product name: Betadex sulfobutyl ether sodium
2) Product specification: Pharmaceutical excipient
3) Registration No.: F20220000553
4) Packaging specification: 25kg/bag/drum
5) MAH: Anhui Poly Pharm. Co., Ltd
Betadex sulfobutyl ether sodium is an excipient and can be used for injectable, oral, ophthalmic, and intranasal drugs as a pharmaceutical one, with special affinity and inclusiveness for nitrogen-containing drugs.
The excipient is usually used in the injections of ziprasidone mesylate, voriconazole, aripiprazole, remdesivir, posaconazole, delafloxacin, and carbamazepine.
This product is an anionic and highly water-soluble β-cyclodextrin derivative developed by the US company Cydex in the 1990s. It is not a single compound component but is composed of isomers with different degrees of substitution and positions/regions from a variety of macromolecules. Therefore, the product can well include drug molecules and form non-covalent compounds to improve the stability, water-solubility, and safety of drugs, lower nephrotoxicity, alleviate drug hemolysis, control drug release speed, and mask adverse odor. Compared with β-cyclodextrin, betadex sulfobutyl ether sodium boasts better solubility in water, milder hemolysis effect, and lower nephrotoxicity, which make it a new pharmaceutical excipient with promising prospects of application.
1、Quality Advantage
1)The product quality is in line with the specifications of USP and EP.
2)The product is featured with low impurity B limit and endotoxin limit and can be widely used in different varieties of drug products.
3)Finished products with customer-specific pH and substitution degree are available.
4)There are dedicated production lines for stable supply. The annual production capacity is over 100 tons.
2、Registration Advantage
The product has undergone API filing/registration in the markets of CN, USA, EU, etc.
① CN registration No.: F20220000553 (result of the joint review with drug products: A)
② US DMF No.: 037814 (status: A)
③ EU certificate No.: CEP 2022-504-Rev 00
Product registration in other markets is ongoing.
Anhui Poly Pharm Co., Ltd, founded in 2018, is in the National High-tech Industrial Development Zone in Anqing. It is a professional supplier of active ingredients, providing chemically synthesized and biosynthesized high-quality ingredients to pharmaceutical, healthcare, and skincare sectors around the world.
Anhui Poly owns 3 API workshops that meet the GMP standards of EU, US, and CN, with 10 production lines and 357 units of multifunctional equipment.
With great R&D capacity, Anhui Poly leverages its techniques to achieve the commercial R&D and production of a collection of products, including contrast agents (meglumine gadoterate, gadoteridol, gadobutrol, iomeprol, iopromide, ioversol, iopamidol, and iodixanol), synthetic biological products (ectoin and salidroside), antineoplastic drugs (cyclophosphamide), other APIs (magnesium hydroxide, apremilast, eucrisa, voriconazole, and memantine hydrochloride), other pharmaceutical (DOTA, betadex sulfobutyl ether sodium, calteridol calcium, and calcobutrol sodium).
Welcome for enquiry, cooperation!
